Safe and secure diagnostic screening procedures to detect genetic abnormalities in the unborn child.

Double marker offers early information about a baby's risk of having Down syndrome (Trisomy 21), Patau syndrome (Trisomy 13) and Edward syndrome (Trisomy18).
First trimester screening, also called the first trimester combined test, has two steps:
Earlier risk assessment of other pregnancy complications like Pre-eclampsia and NTD
The Pentascreen maternal serum test is a first trimester screen that evaluates five maternal serum parameters to assess the risk of a baby being born with Trisomies 21, 18 and 13 and open neural tube defects. It can also predict maternal risk for early and late onset Pre-eclampsia*.
| Detection Rate | False Positive Rate | |
|---|---|---|
| Trisomy 21 | 93% | 3% |
| Early onset PE | 96% | 10% |
| Early onset PE | 77% | 10% |
*PlGF is a predictive marker for early and late onset Pre-eclampsia (PE).

Free β-hCG, AFP, Unconjugated estriol (uE3) Inhibin-A
The Quad Screen is a second trimester screen that assesses the risk of a baby being born with Trisomies 21,18 and 13 and open neural tube defects (ONTDs)
| Test | Detection Rate for Trisomy 21 | False Positive Rate |
|---|---|---|
| Double Marker Screening | 85% | 4-7% |
| First Trimester Quadruple | 95% | 5% |
| Penta Screening without NT | 93% | 5% |
| Quadruple Screening | 81% | 7% |

Noninvasive prenatal testing (NIPT) based on cell-free DNA analysis from maternal blood is a screening test for aneuploidy status for all autosomes including common chromosomal conditions like trisomy 13, trisomy 18, and trisomy 21, sex chromosome abnormalities and select microdeletion syndromes.
The American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) endorse NIPT as having the highest detection rate and lowest false positive rate for the common aneuploidies regardless of maternal age or baseline risk, of all screening options.

Chorionic Villus Sampling( CVS), is done at 10-12 weeks of pregnancy.
You may wish to consider CVS:
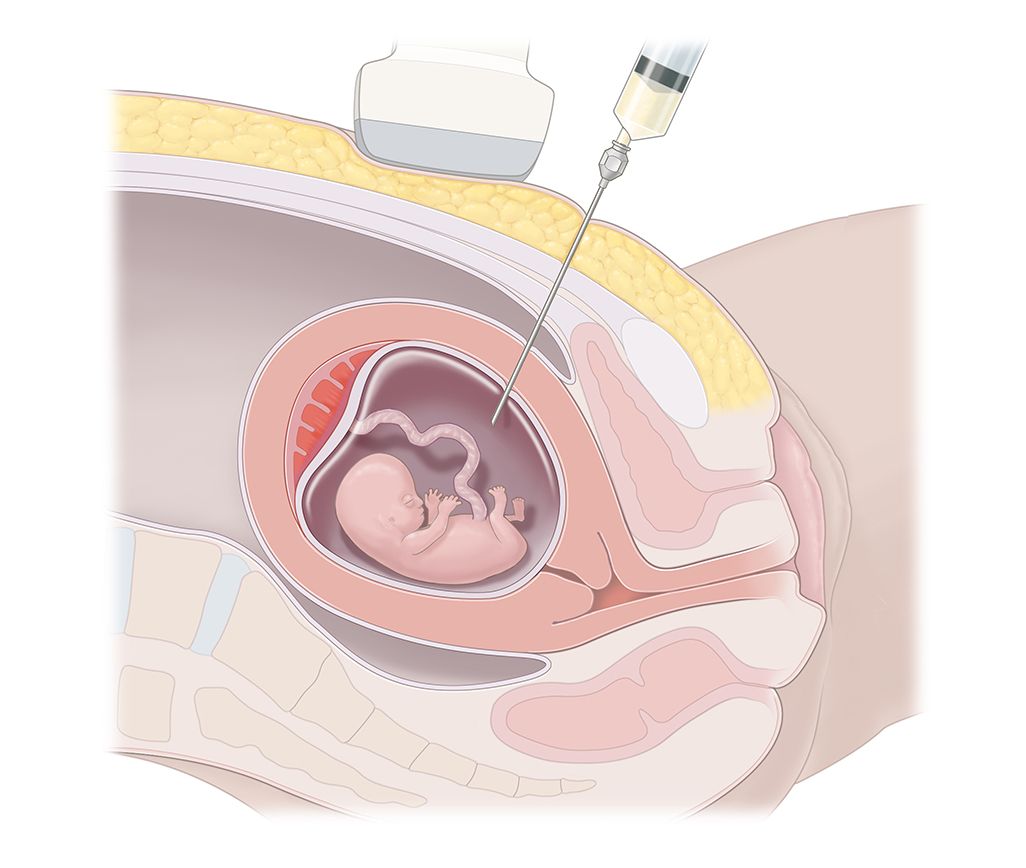
CVS involves taking a tiny amount of the developing placenta, where it is attached to the uterus. The placenta contains tissue that is genetically identical to your baby. CVS may be performed in two ways. Most CVS are performed through the abdomen, but CVS may also be performed through the cervix (neck of the womb). It is performed under an ultrasound guidance to check the positions of both your baby and placenta within the womb.
You would be asked to sign a consent form before the procedure is carried out.
The risk of miscarriage after performing a CVS is approximately 1-2%

Amniocentesis is an invasive procedure performed around 16-18 weeks of pregnancy. In amniocentesis a small amount of amniotic fluid – the water around your baby inside your uterus (womb) is taken for testing.
You may wish to consider amniocentesis if:
The final decision about having any test in pregnancy is yours.
It is an ultrasound guided procedure. You need to have normal breakfast or lunch before the procedure. Try avoiding coffee and tea, as it leads to more movements of the fetus.
After observing the baby’s movement and position the doctor cleans the abdomen with an antiseptic. Under the guidance of ultrasound a small needle is inserted into the amniotic pocket.
This is to avoid any puncture or damage to the baby. About 20 ml of amniotic fluid is taken out. There is no anesthetic required, you will awake throughout the procedure.
You would be asked to sign a consent form before the procedure is carried out.

Health problems? Medical Queries? Send us your questions.
The pain from an amniocentesis procedure is similar to having a blood test from your arm, but there would slight cramps or period like pain during the procedure.
If you are having an amniocentesis for a chromosomal disorder, all chromosomes will be looked at. This means that the test may occasionally detect a problem with the chromosomes which was not expected. If the results show anything abnormal you will be told what the abnormality is and how this will affect your baby. If you are having an amniocentesis for a single gene disorder, only this will be tested for.
The risk of miscarriage after performing a Amniocentesis is approximately 1-2%.